Indication Profiling
We offer broad indication profiling using immunohistochemistry (IHC) based on comprehensive clinical sample collections and tumor microarrays (TMAs) comprising the most relevant tumor indications, including histopathological assessment and scoring of expression (HScore) by a pathologist or semi-automated quantification.
Patient sample profiling using TMAs
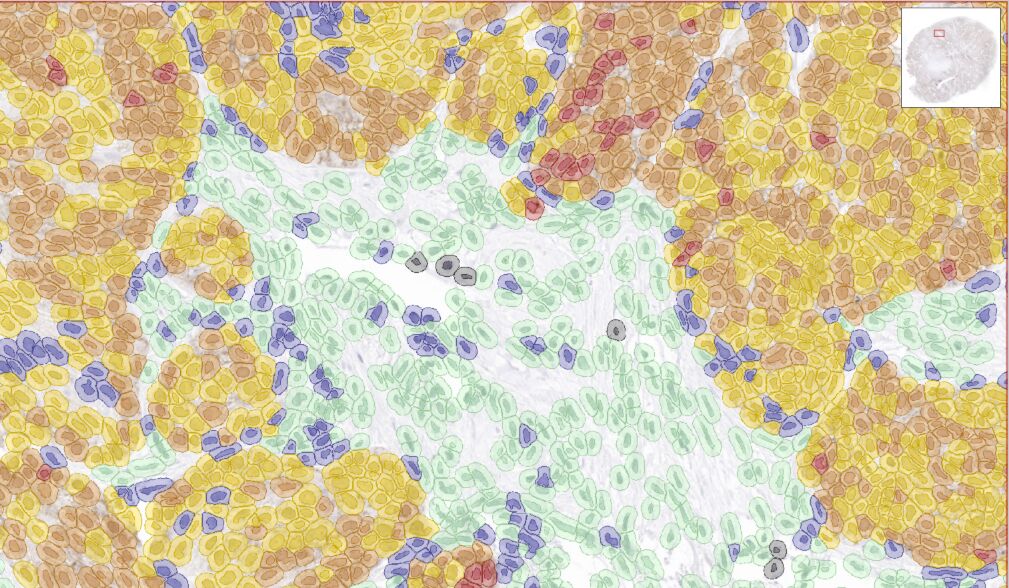
Whole slide scanning & automated quantification
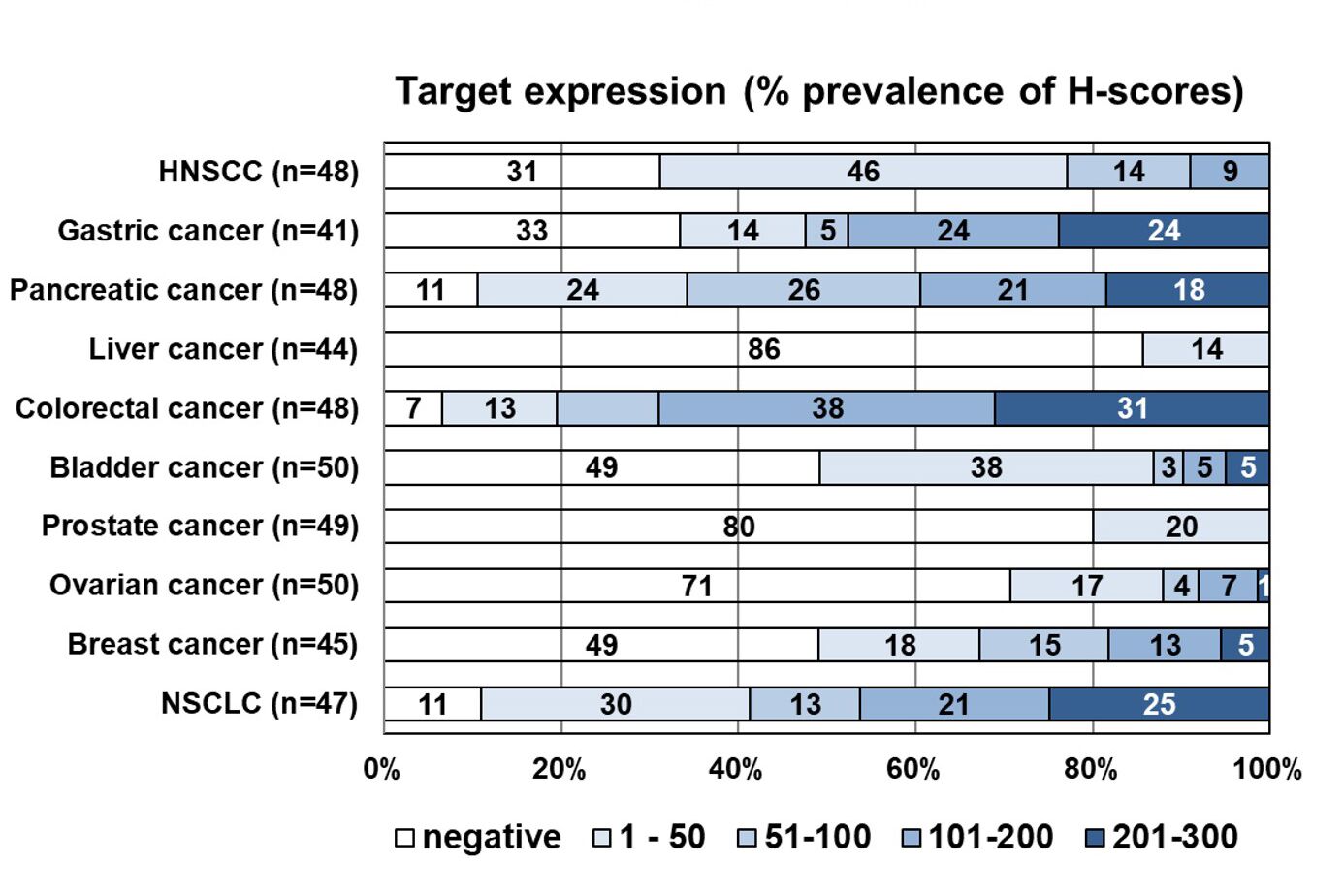
Indication profiling for early clinical trials
Moreover, TMAs are a powerful tool for drug target validation(à1.1.1), enabling assessment of target expression in representative patient samples in a tissue-context. The combination of TMAs with spatial profiling by multicolor immunofluorescence (mIF)(à1.4.1spatial profiling) provides information about cell type specific target expression and cell–cell interactions (e.g., tumor–immune cell interactions).
Clinical Sample Analysis
We offer clinical sample biomarker analysis in accordance with good clinical practice (GCP). We also validate our biomarker assays in accordance with the FDA, bioanalytical method validation guidelines, European Medicines Agency (EMA), and guideline on bioanalytical method validation. Our key expertise is in exploratory biomarker analysis.
Available Platforms:



