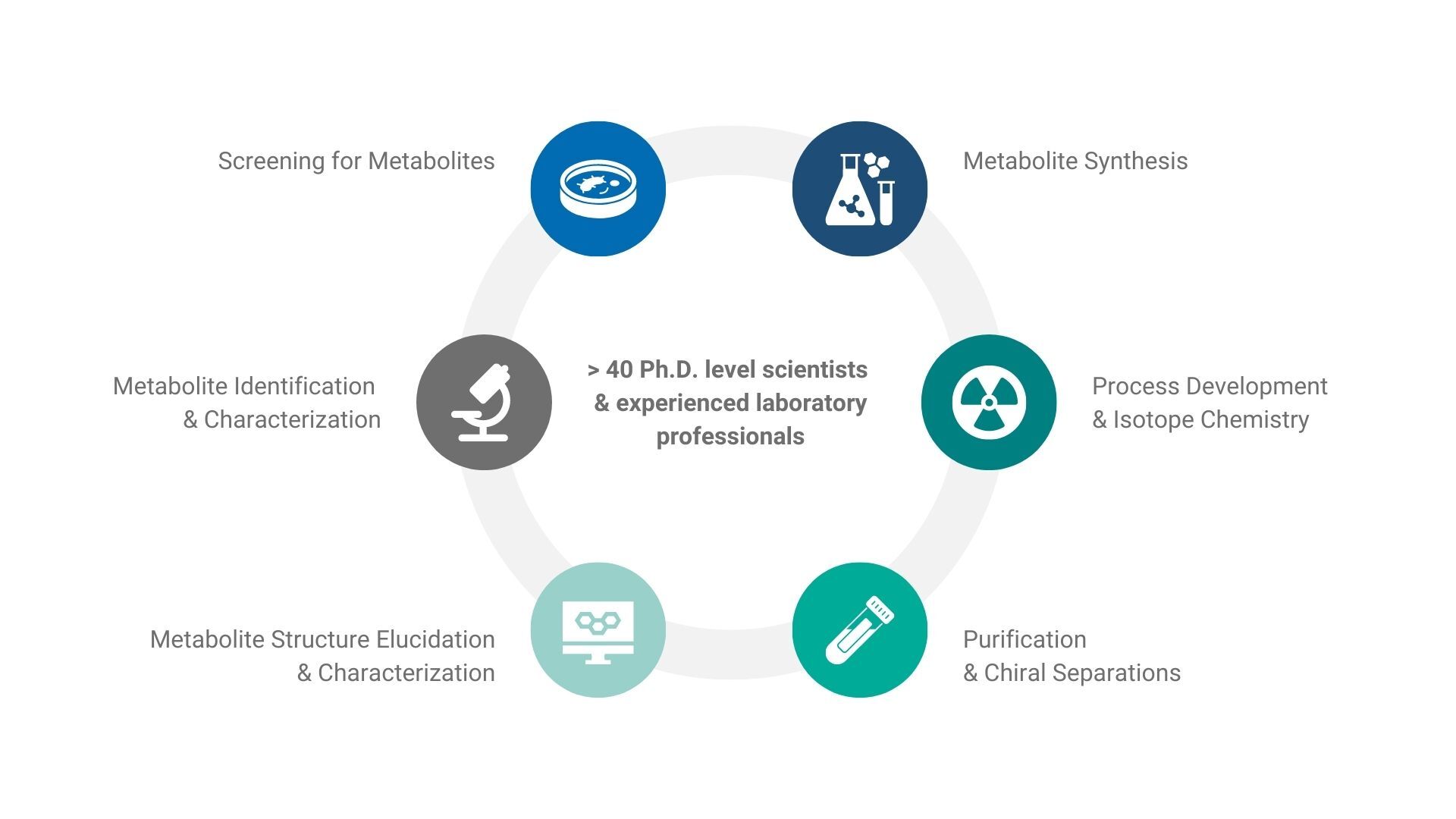
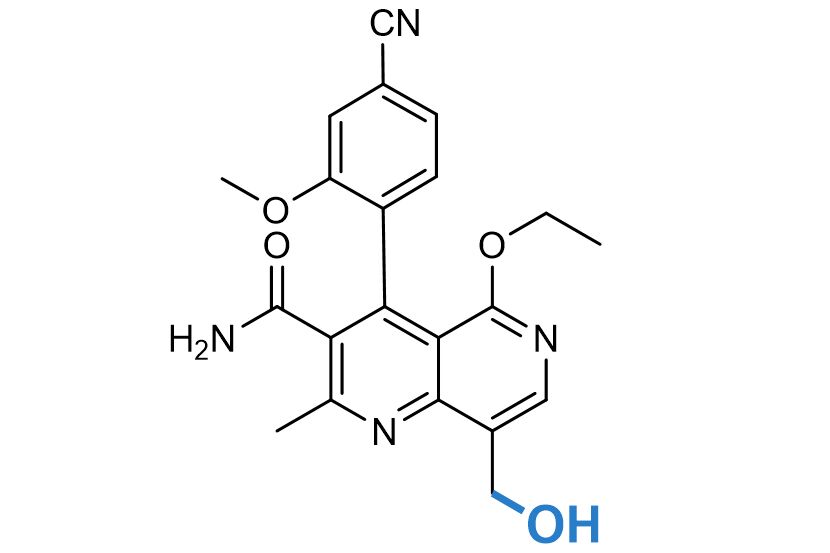
NUVISAN Metabolite Discovery
The NUVISAN metabolite discovery platform combines decades of experience in pharmaceuticals and crop protection to solve your requests regarding drug metabolites. We support your fermentation and biotransformation projects and provide your metabolites for pharmacological activity testing, DMPK, and toxicological investigations, and as standards for analytical methods. We identify, screen for, synthesize, and characterize the metabolites of your interest.