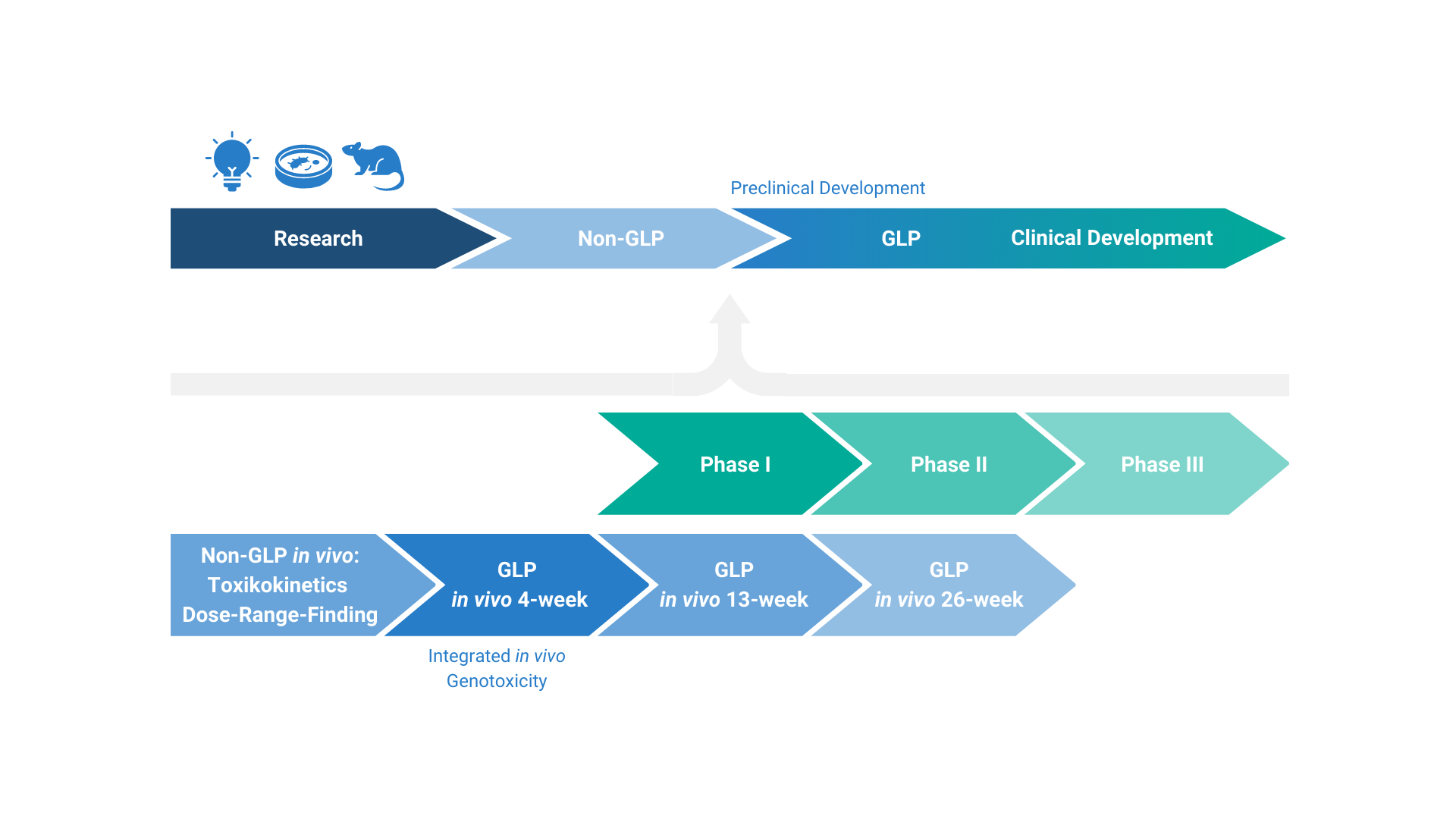
With a comprehensive in vivo safety package, we can be your flexible and reliable partner to guide your product through preclinical and clinical development or REACH testing. We are committed to humane care of animals and the 3Rs principles, and we comply with and even go beyond current legislation. In addition, we always strive for continuous improvements in animal welfare through, for example, our animal welfare development program, as well as our ever-expanding and state-of-the-art in vitro portfolio.
If you are looking to develop a new chemical innovation or a revolutionary new drug, our toxicology services portfolio covers all your needs. Please see in vivo toxicology for pharmaceutical development or in vivo toxicology for chemical development for more information.
Integrated into the company’s expert bioanalysis unit, our toxicokinetics unit makes use of specialized GLP-certified analytical services to navigate your compound through safety packages. In addition, your compound will be accompanied by the same high-quality bioanalytical services from early discovery to preclinical safety, and on to clinical development. Please see GLP bioanalysis and toxicokinetics for more information.
High-quality synthesis is essential for the success of preclinical development, and that holds specifically true for toxicology, as impurities can have a major impact on study outcomes. At NUVISAN, we offer high-quality GMP-compliant synthesis according to international regulatory standards, along with the capacities to develop your compound throughout the value chain—with the same partner all the way. Please see scale-up and special technologies for more information