Currently, animal experiments are indispensable for developing new drug candidates and ensuring their safety and efficacy. For this reason, the performance of animal experiments is one legally required step in drug research and development aimed at reducing the risks of clinical testing in humans. It is expressly provided for in the German Medicines Act, as well as in the applicable drug testing guidelines. In this context, the potential benefit for the treatment of humans and the burden for the animals used in the experiments must be considered in relation to each other and evaluated ethically.
In 2002, animal protection was incorporated into the German constitution and is fundamentally regulated in the Animal Protection Act. This ensures that adequate consideration is given to animal welfare. However, in order to meet the constitutional requirements to maintain and improve human health and the principle of scientific freedom, each individual animal experiment in Germany is subject to careful examination and consideration.
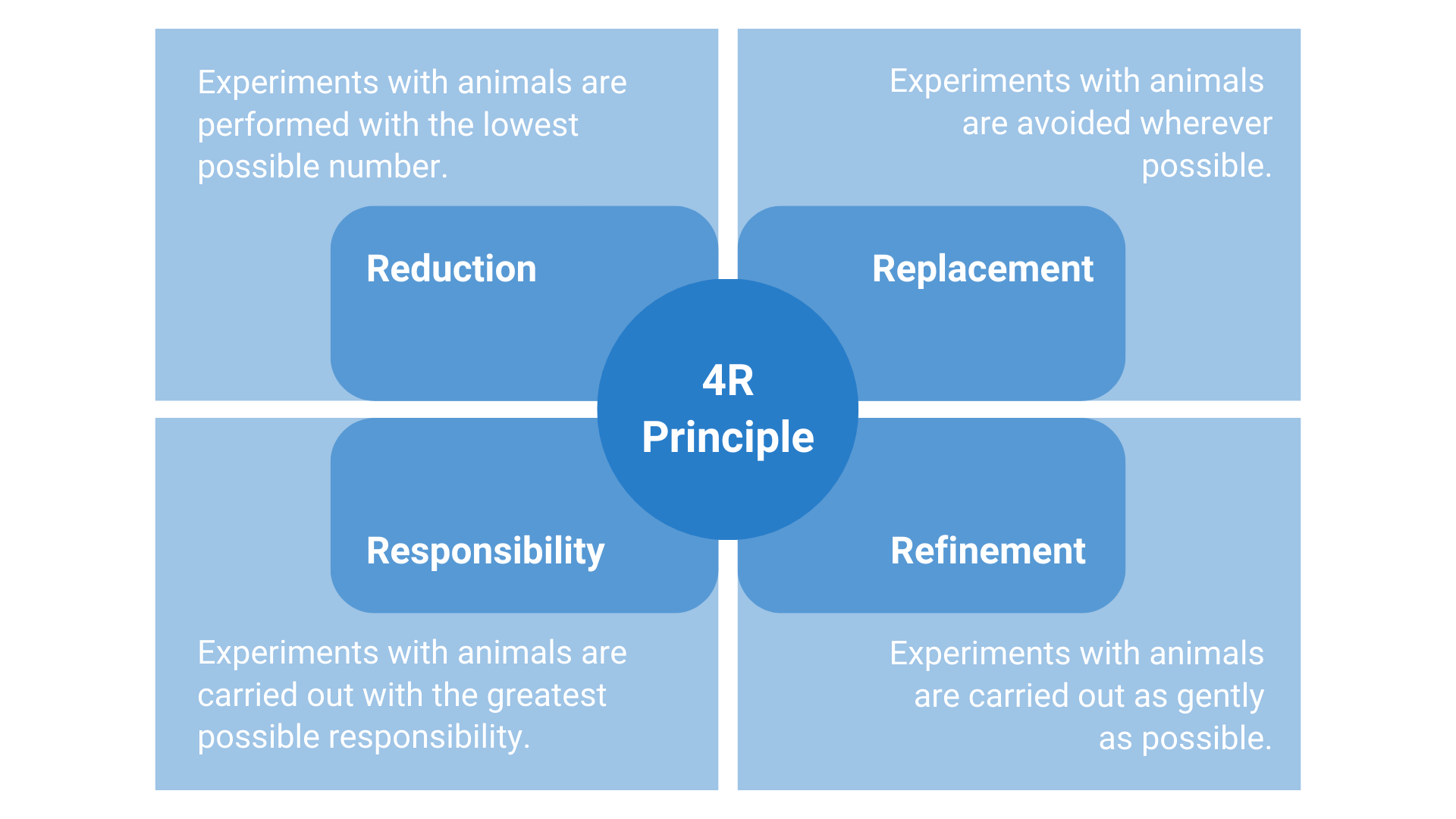
According to the legal regulations for the protection of experimental animals, animal experiments may only be carried out if they have been approved by the competent authorities. For this purpose, a comprehensive application for approval must be submitted in which the planned experiment is described and scientifically and ethically justified. Furthermore, the application has to justify that no alternative (animal-free) methods are available (Replacement), the number of animals used in the experiment is reduced to the minimum (Reduction), and the procedures are as mild as possible (Refinement).
In the sense of the fourth R (Responsibility), NUVISAN ICB has written and adopted an Animal Welfare Declaration. We are committed to the responsible handling of animal experiments and laboratory animals. In addition to complying with legal requirements, we are committed to the continuous further development of experimental and husbandry conditions in the interests of animal welfare. Our high-quality animal welfare requirements are part of the foundation of our excellent research services.