We offer PK/PD and biomarker studies for a broad range of > 100 oncology preclinical models in mice and rats.
- Cell line based syngeneic and xenogeneic models
- Solid and hematological tumor models
- Subcutaneous and orthotopic models (breast, brain, pancreas, liver, kidney)
Analysis/Readout
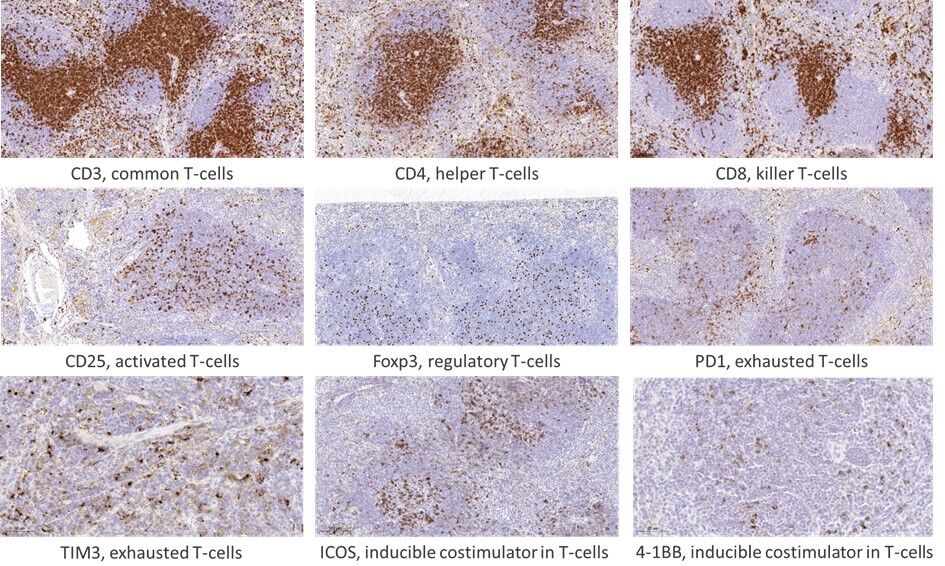
We have established a broad panel of assays for human and mouse targets. A tumor-infiltrating lymphocyte (TIL) panel comprised of over 15 markers for systematic evaluation of TILs in mouse models. Assays for major populations of lymphocytes, macrophages, NK cells, and B cells are available. Protocols were optimized on mouse spleen and used to profile 15 different syngeneic mouse models (data on immune cell infiltration status of syngeneic mouse models is available for customers).
Read more: In vivo pharmacology–oncology platform