SPR is used to determine binding-mediated mass changes of an immobilized target. As one of our flagship tools, SPR combines many advantages of other biophysical methods. It follows label-free molecular interactions in real time. With their high sensitivity, the latest instruments have essentially no lower molecular weight limit of molecules to be studied, and quantification of a large dynamic range from picomolar to weak millimolar affinities is possible. We have long-term experience in characterizing protein–ligand interactions for a broad range of different protein classes (e.g., kinases, proteases, nuclear receptors, E3 ligases, GPCRs, biologics).
Our SPR team consists of more than 10 scientists and lab professionals who use a state-of-the-art SPR platform consisting of four BiacoreTM T/S200 instruments and three BiacoreTM 8K/8K+ instruments.

We perform routine SPR tasks, such as confirmation and quantification of binding, KD determination, and kinetic characterization (rate constants, kon, koff). In addition, we have extensive experience in deciphering the mode-of-action using SPR; enabling us to distinguish between cooperative and competitive bindings and describing the formation of ternary complexes (e.g., PROTACs), including determining the cooperativity factor. We use SPR to study SMOL interactions with proteins, peptides, DNA, and RNA and study the interactions of biomolecules with each other.
Our SPR platform allows us to create mid-scale throughput screens of fragments, focused libraries, selection of nanobodies, and more.
A large part of the success of our biophysical assay development is our in-house protein pipeline, which gives us customized, high-quality protein samples, ensuring reliable SPR data.
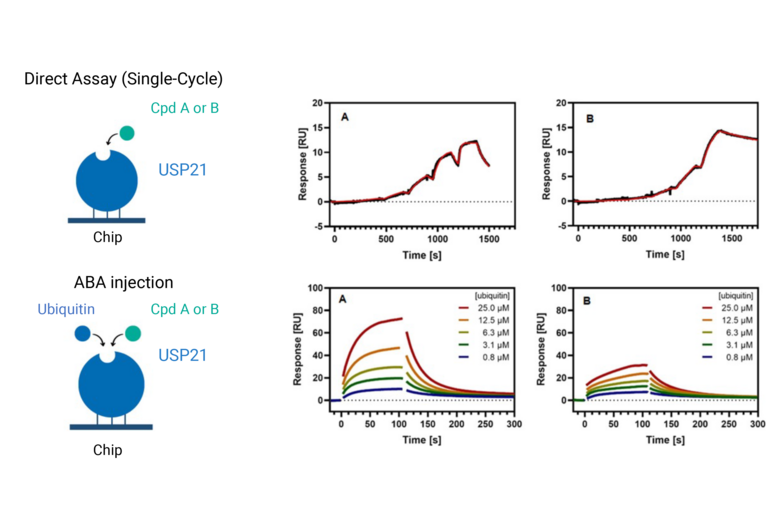
SPR characterization of the competitive binding between compounds A and B with ubiquitin for USP21. Reference: Göricke, F., et al., J Med Chem (2023)


